
Procedures for Importing Specially Controlled Chemicals Under Decree No. 24/2026/ND-CP (Effective from January 17, 2026)
Date Submitted: 05/02/2026 03:31 PM
Xem chi tiết![🔥 [BREAKING NEWS] Decree 46/2026/ND-CP Officially “Terminates” the Self-Declaration Mechanism – A New Era of Food Safety Management](https://songwinlog.net/thumbs/455x500x1/upload/news/them-tieu-de-phu-9121.png)
🔥 [BREAKING NEWS] Decree 46/2026/ND-CP Officially “Terminates” the Self-Declaration Mechanism – A New Era of Food Safety Management
Date Submitted: 04/02/2026 11:26 AM
Xem chi tiết
DETAILED GUIDELINES ON CLASSIFICATION RULES FOR IN VITRO DIAGNOSTIC MEDICAL DEVICES (IVD)
Date Submitted: 30/01/2026 02:49 PM
Xem chi tiết
GUIDELINES FOR CLASSIFYING MEDICAL DEVICES (Circular 05/2022/TT-BYT): SPECIAL CLASSIFICATION RULES
Date Submitted: 28/01/2026 01:55 PM
Xem chi tiết
DETAILED GUIDANCE: RULES FOR CLASSIFICATION OF ACTIVE MEDICAL DEVICES UNDER CIRCULAR 05/2022/TT-BYT
Date Submitted: 26/01/2026 01:57 PM
Xem chi tiết
GUIDELINES FOR CLASSIFICATION OF INVASIVE MEDICAL DEVICES (According to Circular No. 05/2022/TT-BYT)
Date Submitted: 23/01/2026 02:49 PM
Xem chi tiết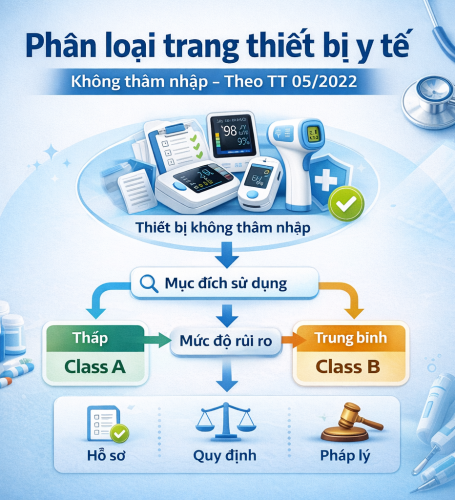
DETAILED GUIDELINES: CLASSIFICATION RULES FOR NON-INVASIVE MEDICAL DEVICES (In accordance with Circular No. 05/2022/TT-BYT)
Date Submitted: 21/01/2026 02:43 PM
Xem chi tiết
CLASSIFICATION OF MEDICAL DEVICES According to Decree No. 98/2021/ND-CP & Circular No. 05/2022/TT-BYT
Date Submitted: 19/01/2026 11:48 AM
Xem chi tiết
Declaration of Applied Standards – Mandatory Procedure for Class A & B Medical Devices
Date Submitted: 16/01/2026 02:02 PM
Xem chi tiết
GUIDELINES ON DECLARATION OF APPLICABLE STANDARDS FOR MEDICAL DEVICES
Date Submitted: 14/01/2026 10:53 AM
Xem chi tiết










