Correctly determining the classification of medical devices is the first and most critical step in the process of obtaining circulation permits and import licenses.
For Active Medical Devices, the classification rules have specific characteristics that require enterprises to fully understand in order to ensure legal compliance.
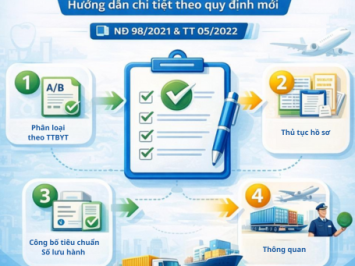
This article provides an overview and an in-depth explanation of Rules 9, 10, 11, and 12 under Circular 05/2022/TT-BYT, helping you accurately determine the risk level of your product.
1. Overview and Key Definitions
Before reviewing the specific rules, it is essential to clearly understand the subject matter.
What are Active Medical Devices?
According to current regulations, Active Medical Devices are medical devices that operate using electrical energy or any other source of energy other than energy generated directly by the human body or gravity.
Common examples include:
X-ray machines, ventilators, patient monitoring systems, electronic diagnostic equipment, etc.
Why Is Accurate Classification Important?
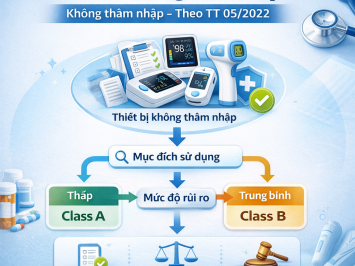
Medical device classification (Class A, B, C, or D) determines the applicable regulatory pathway:
-
Class A, B: Declaration of applied standards
-
Class C, D: Registration for circulation (more complex procedures)
2. Detailed Classification Rules for Active Medical Devices
Below are the detailed contents of Group C Rules (Rules 9, 10, 11, and 12) in accordance with Circular 05/2022/TT-BYT:
Rule 9: Classification of Active Therapeutic Medical Devices
This rule applies to devices used for treatment or therapeutic support that utilize energy.
Class B:
This is the default classification for all active therapeutic devices intended to distribute or exchange energy, unless they fall under higher-risk conditions below.
Class C (Higher Risk):
-
Devices that distribute or exchange energy to or from the human body in a manner that may pose risks, including the emission of ionizing radiation, taking into account the nature, density, and site of energy application.
-
Active devices intended to control, monitor, or directly influence the performance of Class C active therapeutic devices.
Rule 10: Classification of Active Medical Devices for Diagnosis
This is the most complex rule group due to the wide variety of diagnostic imaging and functional examination devices.
Class A:
-
Devices intended to illuminate the patient’s body using light in the visible or near-infrared spectrum.
Class B:
-
Devices intended to supply energy absorbed by the human body (excluding Class A illumination devices).
-
Devices used to image the distribution of radiopharmaceuticals in the body.
-
Devices used for direct diagnosis or monitoring of physiological processes.
Class C:
-
Devices used to monitor physiological parameters where variations could result in immediate danger to the patient (e.g., cardiac activity, respiration, central nervous system).
-
Devices used for diagnosis in clinical situations where the patient is in a critical condition.
-
Devices emitting ionizing radiation and used for diagnostic or interventional X-ray procedures, including devices that control or monitor such equipment.
Rule 11: Devices for Administering or Removing Substances to/from the Body
This rule applies to equipment such as infusion pumps, dialysis machines, suction devices, etc.
Class B:
-
The default classification for all devices in this group.
Class C:
-
If the device presents a potential danger to the patient due to:
-
The nature of the substance involved
-
The part of the body concerned
-
The method and route of administration or removal
-
Rule 12: Classification of Other Active Medical Devices
Class A:
-
All active medical devices not covered under Rules 9, 10, and 11 are classified as Class A.
3. Your Trusted Partner for Medical Device Import & Logistics – Songwin International Logistics
Understanding classification rules is only the first step. To legally import and circulate medical devices in Vietnam, enterprises require a well-structured logistics, customs, and regulatory compliance process.
Songwin International Logistics Vietnam proudly provides comprehensive solutions for the medical device supply chain.
Why Choose Songwin International Logistics?
-
Regulatory Expertise: Our specialists have in-depth knowledge of Circular 05/2022/TT-BYT, Decree 98/2021/NĐ-CP, and relevant implementing regulations. We support accurate classification and minimize compliance risks.
-
End-to-End Services: International transportation (Sea/Air), customs clearance, import licensing, and medical device circulation registration.
-
Safety & Professionalism: Medical devices require strict handling and storage standards. Songwin ensures your cargo is managed with the highest level of care and professionalism.
If you are facing challenges in classifying active medical devices or need a reliable logistics partner, Songwin International Logistics is ready to accompany you.
📞 Contact us today for a free consultation!
SONGWIN INTERNATIONAL LOGISTICS VIETNAM CO., LTD
📍 Address: 344 Nguyen Trong Tuyen Street, Tan Son Hoa Ward, Ho Chi Minh City, Vietnam
📞 24/7 Hotline: +84 83 681 3969 – +84 373 262 105
📧 Email: Sales2@songwinlog.com
Songwin Logistics – Trust Builds the Brand, Professionalism Creates Success.