Accurate classification of medical devices is the first and most critical step in the process of obtaining circulation licenses and import permits. For invasive medical devices, the risk level is generally higher; therefore, the classification rules under Circular No. 05/2022/TT-BYT are stricter and more complex.
This article summarizes fundamental knowledge and provides an in-depth analysis of Rules 5, 6, 7, and 8, which apply specifically to this group of devices.
I. OVERVIEW AND BASIC CONCEPTS
Before examining specific rules, enterprises must clearly understand the definitions related to invasiveness and duration of use in order to apply the correct classification rules.
1. Definition of “Invasive”
-
Invasive via a body orifice: Devices that enter the body through natural openings (nose, mouth, ear, etc.) without surgical incision.
-
Surgically invasive: Devices that enter the body through the body surface by means of surgical intervention or within a surgical context.
2. Definition of “Invasive Medical Devices”
Invasive medical devices are medical devices that partially or wholly enter the human body through a body orifice or through the body surface, including:
-
Implantable medical devices
-
Surgically invasive medical devices
-
Medical devices invasive via natural body orifices
-
Medical devices invasive through the body surface
Implantable medical devices are devices implanted into the human body through surgery or used to replace an epithelial surface or ocular surface for the purpose of maintaining organ function after implantation, including devices partially introduced into the body via surgery for at least 30 days.
Surgically invasive medical devices are devices introduced into the body through the body surface with surgical assistance, including those that do not enter via natural body orifices.
3. Regulations on Duration of Use
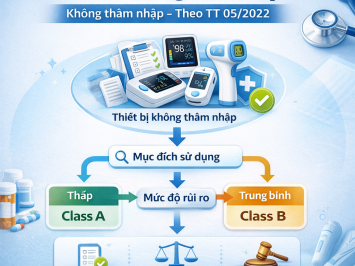
The duration of contact with the human body is a key factor in determining the risk class (Class A, B, C, or D):
-
Transient use: Continuous use for less than 60 minutes
-
Short-term use: Continuous use from 60 minutes to 30 days
-
Long-term use: Continuous use for more than 30 days
II. CLASSIFICATION RULES FOR INVASIVE MEDICAL DEVICES
Below are details from Appendix I – Part B of Circular No. 05/2022/TT-BYT regarding the classification of invasive medical devices.
Rule 5: Medical Devices Invasive via a Body Orifice (Non-surgical)
This rule applies to devices introduced into the body through natural openings (mouth, nose, ear, etc.) and not connected to active medical devices (or only connected to Class A active devices).
| Duration of Use | Basic Classification | Exceptions |
|---|---|---|
| Transient (< 60 minutes) | Class A | Upgraded to Class B if used on body surfaces, the eyeball, or if capable of being absorbed by mucous membranes |
| Short-term (60 min – 30 days) | Class B | Downgraded to Class A if used in the oral cavity to pharynx, ear canal to eardrum, or nasal cavity |
| Long-term (> 30 days) | Class C | Downgraded to Class B if used in the above regions and not absorbable by mucous membranes |
Special note: All non-surgically invasive devices connected to active medical devices of Class B or higher shall be classified as Class B.
Rule 6: Surgically Invasive Medical Devices – TRANSIENT USE
(Duration: Less than 60 minutes)
General principle: Mostly classified as Class B.
Exceptions:
-
Class A: Reusable surgical instruments (e.g., scalpels, scissors, forceps)
-
Class C: Devices used to:
-
Supply energy in the form of ionizing radiation
-
Produce biological effects or be wholly/partially absorbed
-
Deliver medicinal products via potentially hazardous delivery systems
-
-
Class D: Devices that:
-
Come into direct contact with the central nervous system
-
Diagnose, monitor, or correct defects of the heart or central circulatory system (direct contact)
-
Rule 7: Surgically Invasive Medical Devices – SHORT-TERM USE
(Duration: 60 minutes to 30 days)
General principle: Mostly classified as Class B.
Exceptions (higher risk):
-
Class C: Devices used to:
-
Administer medicinal products
-
Undergo chemical changes in the body (excluding dental devices)
-
Supply ionizing radiation energy
-
-
Class D: Devices that:
-
Produce biological effects or are wholly/partially absorbed
-
Come into direct contact with the central nervous system
-
Diagnose, monitor, or correct defects of the heart or central circulatory system (direct contact)
-
Rule 8: Surgically Invasive Medical Devices – LONG-TERM USE & IMPLANTABLE DEVICES
(Duration: Over 30 days or permanent implantation)
General principle: Mostly classified as Class C.
Exceptions:
-
Downgraded to Class B: Dental devices (e.g., bridges, crowns)
-
Upgraded to Class D (Highest risk): Devices that:
-
Directly contact the heart, central circulatory system, or central nervous system
-
Support or sustain life
-
Are active medical devices
-
Produce biological effects or are wholly/partially absorbed
-
Deliver medicinal products
-
Undergo chemical changes in the body (excluding dental devices)
-
Special case: Breast implants
-
III. MEDICAL DEVICE CLASSIFICATION & IMPORT SOLUTIONS WITH SONGWIN LOGISTICS
Incorrect classification can cause serious delays, administrative penalties, or even revocation of circulation numbers. For invasive devices (Rules 5–8), the boundary between Class B, C, and D is often subtle and heavily dependent on the intended use.
Songwin Logistics is proud to be a trusted partner, providing end-to-end solutions for medical device enterprises.
Why Choose Songwin Logistics?
-
Deep expertise: Specialists with thorough knowledge of Decree No. 98/2021/NĐ-CP and Circular No. 05/2022/TT-BYT
-
Accuracy: Precise risk classification consulting to optimize registration dossiers
-
Speed: Shortened licensing and customs clearance timelines for faster market access
Our Key Services:
-
Medical device classification consulting (Class A, B, C, D)
-
Circulation registration and declaration of applicable standards
-
Import license and advertising license applications
-
Comprehensive logistics services: international transportation and professional medical customs clearance
👉 Contact us now for a FREE medical device classification consultation!
SONGWIN INTERNATIONAL LOGISTICS VIETNAM CO., LTD.
📍 Address: 344 Nguyen Trong Tuyen Street, Tan Son Hoa Ward, Ho Chi Minh City
📞 24/7 Hotline: +84 83 681 3969 – +84 373 262 105
📧 Email: Sales2@songwinlog.com
Songwin Logistics – Credibility Builds the Brand, Professionalism Drives Success.