Correctly identifying the classification of a medical device is the first and most critical step in the process of importing and placing products on the Vietnamese market.
Errors in classification may not only lead to application rejection but also pose serious legal risks for enterprises.
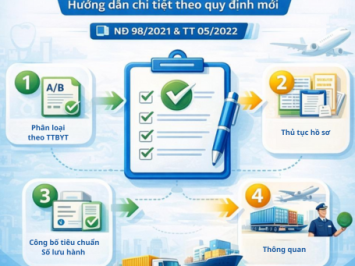
This article summarizes the core regulations on definitions, principles, and classification rules based on Decree No. 98/2021/ND-CP and Circular No. 05/2022/TT-BYT.
1. What is a Medical Device?
(According to Article 2 of Decree No. 98/2021/ND-CP)
Under Article 2 of Decree No. 98/2021/ND-CP, medical devices include instruments, apparatuses, materials, implants, in vitro diagnostic reagents and calibrators, and software that simultaneously meet the following conditions:
1. Intended for use, either alone or in combination, as specified by the product owner, for human beings for one or more of the following purposes:
-
Diagnosis, prevention, monitoring, treatment, or alleviation of disease, or compensation for injury or disability;
-
Investigation, replacement, modification, or support of anatomy or physiological processes;
-
Life support or life-sustaining purposes;
-
Control of conception;
-
Disinfection and sterilization of medical devices (including testing chemicals);
-
Providing information for diagnosis, monitoring, or treatment through examination of specimens derived from the human body.
Note:
Medical devices do not achieve their primary intended action by pharmacological, immunological, or metabolic means, although such mechanisms may assist their function.
2. Medical Device Classification Levels
(According to Article 4 of Decree No. 98/2021/ND-CP)
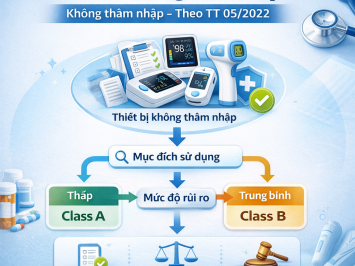
Medical devices are classified based on the potential risks related to their technical design and manufacturing. There are four risk classes as follows:
| Group | Class | Risk Level | Typical Examples |
|---|---|---|---|
| Group 1 | A | Low risk | Cotton gauze, examination gloves, wheelchairs |
| Group 2 | B | Low–medium risk | Syringes, infusion sets, suction devices |
| Group 2 | C | Medium–high risk | X-ray machines, electrosurgical units, patient monitors |
| Group 2 | D | High risk | Heart valves, coronary stents, implantable devices |
3. Principles of Medical Device Classification
(According to Article 5 of Decree No. 98/2021/ND-CP)
Medical device classification must strictly comply with the following principles:
-
Risk-based classification: Devices are classified according to their level of potential risk.
-
Highest risk principle: If a medical device may fall into multiple risk classes due to multiple intended uses or functions, the highest applicable risk class shall be applied.
-
Authority: Classification must be conducted by an entity that declares applicable standards or registers the medical device for circulation.
4. Detailed Classification Rules under Circular No. 05/2022/TT-BYT
Circular No. 05/2022/TT-BYT provides detailed guidance for implementing Decree No. 98.
In particular, Appendix I explains terminology and sets out specific classification rules.
A. Key Terminology (Appendix I)
To ensure accurate classification, enterprises must clearly understand the following terms:
-
Active medical device: A device that operates using electrical energy or any other energy source other than energy generated by the human body or gravity.
-
Invasive medical device: A device that enters the body, either partially or completely, through a body orifice or the body surface.
-
Surgically invasive medical device: A device that enters the body through surgical intervention or in a surgical context.
B. Overview of Classification Rules (Appendix I)
Circular No. 05 groups classification rules based on device characteristics:
1. Rules for NON-INVASIVE medical devices:
-
Applicable to devices that only contact intact skin, mucous membranes, or do not directly contact the body.
-
Most are classified as Class A or B, except those intended to store blood or body fluids for reintroduction into the body (which may be Class C).
2. Rules for INVASIVE medical devices:
-
Classification depends on duration of contact
(short-term < 60 minutes, temporary < 30 days, long-term > 30 days)
and site of invasion. -
Devices invasive through natural body orifices generally pose lower risk than surgically invasive devices.
3. Rules for ACTIVE medical devices:
-
Include diagnostic and therapeutic devices.
-
Devices that supply energy or substances to the human body at potentially hazardous levels, or that control vital physiological processes, are usually classified as Class C or D.
4. ADDITIONAL rules:
-
Specific provisions apply to special-purpose devices such as sterilization equipment, contraceptive devices, X-ray imaging systems, etc.
5. Comprehensive Solutions from Songwin International Logistics Vietnam
Are you facing difficulties in classifying medical devices or navigating complex customs and regulatory procedures?
Songwin International Logistics Vietnam is proud to provide end-to-end logistics and specialized legal consulting services for the medical device industry. With a team deeply knowledgeable in Decree No. 98 and Circular No. 05, we are committed to helping enterprises optimize time, cost, and compliance.
Our key services include:
-
✅ Medical device classification consulting: Accurate classification into Classes A, B, C, or D to ensure valid documentation from the outset.
-
✅ Import license & circulation registration: Support for declaration of applicable standards (Class A, B) and circulation registration (Class C, D).
-
✅ International transportation & customs clearance: Professional handling of medical shipments with standard-compliant storage (cool and cold chain warehouses).
-
✅ Tax & HS code consulting: Optimization of import duties and VAT for medical devices.
Don’t let regulatory barriers slow down your business opportunities.
Let Songwin be your trusted partner in bringing medical devices to the Vietnamese market quickly, safely, and compliantly.