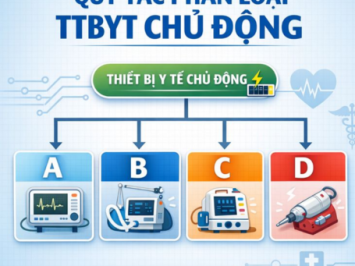
According to Circular No. 05/2022/TT-BYT
The classification of In Vitro Diagnostic Medical Devices (IVDs) is the first and most critical step for enterprises to determine applicable import procedures, including:
-
Declaration of Applicable Standards for Class A or B, or
-
Registration for Circulation for Class C or D devices.
Pursuant to Circular No. 05/2022/TT-BYT, IVDs are classified based on risk levels from low to high (Class A, B, C, D), following 07 specific classification rules as outlined below:
1. CLASSIFICATION RULES FOR HIGH-RISK GROUP (CLASS D)
Class D medical devices represent the highest risk level to individuals and public health.
Rule 1: Detection of Dangerous Infectious Agents
IVDs are classified as Class D if they are used for:
-
Detecting the presence of or exposure to infectious agents in blood, tissues, cells, etc., for the purpose of assessing suitability for blood transfusion or transplantation.
-
Detecting life-threatening pathogens that are often incurable and have a high risk of transmission.
Rule 2: Blood Grouping and Tissue Typing (Exceptions)
Most IVDs used to determine blood groups or tissue compatibility for transfusion or transplantation are classified as Class C, EXCEPT the following systems, which are classified as Class D:
-
ABO system: A (ABO1), B (ABO2), AB (ABO3)
-
Rhesus system: RH1 (D), RH2 (C), RH3 (E), RH4 (c), RH5 (e)
-
Kell system: Kel1 (K)
-
Kidd system: JK1 (Jka), JK2 (Jkb)
-
Duffy system: FY1 (Fya), FY2 (Fyb)
2. CLASSIFICATION RULES FOR MEDIUM-HIGH RISK GROUP (CLASS C)
This group includes critical diagnostic tests where incorrect results may cause serious consequences.
Rule 3: Infectious Diseases, Genetic Testing, and Screening
IVDs are classified as Class C if they are used for:
-
Detection of sexually transmitted diseases (STDs) (e.g., Chlamydia, Gonorrhea).
-
Detection of infectious agents in cerebrospinal fluid or blood with limited transmissibility (e.g., Meningitis, Cryptococcus fungi).
-
Detection of agents where false results may cause death or severe disability (e.g., CMV, Methicillin-resistant Staphylococcus aureus).
-
Prenatal screening to determine immune status against infectious diseases (Rubella, Toxoplasmosis).
-
Determination of infectious or immune status where incorrect results may lead to life-threatening risks due to inappropriate treatment decisions (e.g., Enterovirus, CMV, HSV testing in transplant patients).
-
Cancer screening and disease staging (personalized medicine).
-
Human genetic testing.
-
Monitoring drug or substance concentrations where inaccurate results may cause immediate danger (cardiology, coagulation).
-
Monitoring treatment of life-threatening infectious diseases (HIV, HCV).
-
Screening for congenital fetal disorders (Down syndrome, spina bifida).
Rule 4: Self-Testing and Point-of-Care Testing (POCT)
-
Self-tests: Classified as Class C.
-
Note: If results are for reference only and require confirmatory laboratory testing, the device is classified as Class B.
-
-
POCT devices: Blood gas and blood glucose testing devices are classified as Class C.
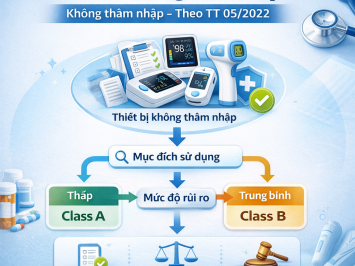
3. CLASSIFICATION RULES FOR MEDIUM-LOW RISK GROUP (CLASS B)
Rule 6: Exclusion Principle
IVDs that do not fall under Rules 1, 2, 3, 4, or 5 are classified as Class B.
Examples: Conventional biochemistry reagents, analyzers that do not perform Class C or D parameters, etc.
Rule 7: Control Materials
Control materials (Controls) that are not assigned quantitative or qualitative values are classified as Class B.
4. CLASSIFICATION RULES FOR LOW-RISK GROUP (CLASS A)
Rule 5: Auxiliary Products
IVDs are classified as Class A if they include:
-
Products supporting the testing process (buffers, washing solutions, etc.).
-
Devices designated for shared use within in vitro procedures.
-
Specimen containers (test tubes, sample vials, etc.).
💡 LOGISTICS & IMPORT CONSULTING SOLUTIONS FOR MEDICAL DEVICES
From SONGWIN INTERNATIONAL LOGISTICS
Incorrect classification may result in rejection of circulation registration dossiers or customs clearance issues. To ensure your medical device shipments arrive safely, legally, and on schedule, partner with Songwin International Logistics Vietnam.
We are proud to be a specialized logistics provider for the healthcare sector, offering:
✅ Regulatory & Classification Consulting
Experienced experts with in-depth knowledge of Circular 05/2022/TT-BYT and Decree 98/2021/NĐ-CP, ensuring accurate classification and compliant documentation.
✅ Multimodal International Transportation
A global agent network capable of handling temperature-controlled medical shipments (cold chain, dry ice) with maximum safety.
✅ Fast Customs Clearance
Efficient handling of inspections, valuation consultations, and clearance procedures to bring products to market quickly.
✅ Cost-Optimized Solutions
Comprehensive Door-to-Door logistics services at competitive pricing.
Don’t let regulatory procedures slow down your business opportunities!
Contact Songwin International Logistics today for free consultation on classification and logistics quotations:
SONGWIN INTERNATIONAL LOGISTICS VIETNAM CO., LTD.
📍 Address: 344 Nguyen Trong Tuyen Street, Tan Son Hoa Ward, Ho Chi Minh City, Vietnam
📞 24/7 Hotline: +84 83 681 3969 – +84 373 262 105
📧 Documentation Email: Sales2@songwinlog.com