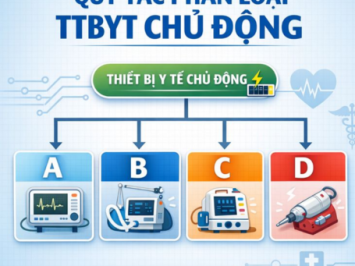
Accurate classification of medical devices is the first and most critical step in the process of importing and legally placing products on the Vietnamese market.
Under Circular No. 05/2022/TT-BYT, in addition to the general rules based on invasiveness and risk level, the group of Other Classification Rules (Part D) provides very specific guidance for special categories such as combination devices, devices of biological origin, and disinfection/sterilization products.
Below are the details of Rules 13, 14, 15, and 16, helping businesses correctly determine device classes (A, B, C, or D).
1. Rule 13: Medical Devices Combined with Medicinal Substances
This group carries a high level of risk due to the combination of a device and a pharmaceutical substance.
Classification: Class D
Applicable conditions: Medical devices that incorporate a medicinal substance, where the substance plays a supporting role in the device’s function on or in the human body.
Note: Determining whether the medicinal substance has a primary or auxiliary role is the key factor in distinguishing a medical device from a pharmaceutical product.
2. Rule 14: Medical Devices of Animal or Microbial Origin
For devices with biological origins, the level of risk depends on the method of contact and the source of raw materials.
Devices classified as Class D (Highest risk) include those containing:
– Animal cells, tissues, or derivatives that are not capable of independent growth.
– Cells, tissues, or derivatives of bacterial origin or produced through recombinant technology.
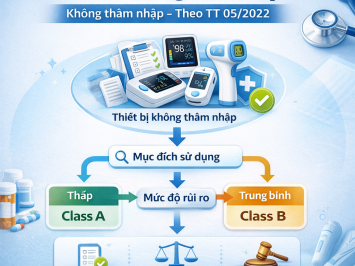
Devices classified as Class A (Lowest risk)
Condition: Devices containing animal tissue or tissue derivatives (not capable of independent growth) that only come into contact with intact skin.
3. Rule 15: Medical Devices Used for Disinfection and Sterilization
Classification depends on the stage of use in the cleaning and sterilization process.
| Intended Use | Classification |
|---|---|
| Used to sterilize medical devices | Class C |
| Used for disinfection of medical devices (final stage) | Class C |
| Used for contact lenses (disinfection, cleaning, soaking, rinsing, moisturizing) | Class C |
| Used for disinfection (prior to sterilization) | Class B |
| Used for disinfection (prior to the final disinfection stage) | Class B |
4. Rule 16: Contraceptive Devices and Devices for Prevention of Sexually Transmitted Diseases
This rule divides devices into two risk levels based on invasiveness and duration of use:
Class C: Devices used for contraception or prevention of sexually transmitted diseases with non-invasive, common use
(Example: Condoms)
Class D: Invasive devices used for long-term application or implantation
(Examples: Intrauterine devices (IUDs), contraceptive implants)
Logistics & Import Procedure Solutions for Medical Devices with Songwin International Logistics Vietnam
Understanding Circular 05/2022/TT-BYT is only the starting point. To safely, quickly, and legally bring medical devices into Vietnam, you need a logistics partner with deep expertise in the medical sector.
Songwin International Logistics Vietnam is proud to provide comprehensive logistics and customs clearance solutions for medical devices.
Why Choose Songwin Logistics?
In-depth legal expertise: Our team thoroughly understands classification rules (A, B, C, D), import license requirements, circulation numbers, and declarations of applied standards under the latest regulations.
Accurate classification consulting: We assist clients in reviewing technical dossiers and product catalogues to correctly determine HS codes and risk levels, minimizing the risk of administrative penalties.
Safe transportation: Standardized logistics processes ensuring strict temperature control and proper storage conditions for sensitive medical devices (such as diagnostic reagents and devices containing medicinal substances).
Fast customs clearance: Efficient handling of inspections, customs valuation consultations, and clearance procedures to ensure on-time delivery.
Our Services Include:
– International sea and air freight
– Full-package customs declaration services for medical devices
– Consulting on registration and declaration procedures for medical device circulation
– Domestic transportation and warehousing services
Don’t let administrative barriers slow down your business opportunities.
📩 Contact Songwin International Logistics Vietnam today for a free consultation on medical device import procedures!
SONGWIN INTERNATIONAL LOGISTICS VIETNAM CO., LTD
📍 Address: 344 Nguyen Trong Tuyen Street, Tan Son Hoa Ward, Ho Chi Minh City, Vietnam
📞 24/7 Hotline: 083.681.3969 – 0373.262.105
📧 Email for document submission: Sales2@songwinlog.com
Songwin Logistics – Trust Builds the Brand, Professionalism Creates Success.