In the business and importation of medical devices, incorrect risk classification not only causes delays in licensing procedures but also exposes enterprises to significant administrative penalties.
Currently, Circular No. 05/2022/TT-BYT issued by the Ministry of Health is the highest-level legal document providing detailed guidance on medical device classification in Vietnam.
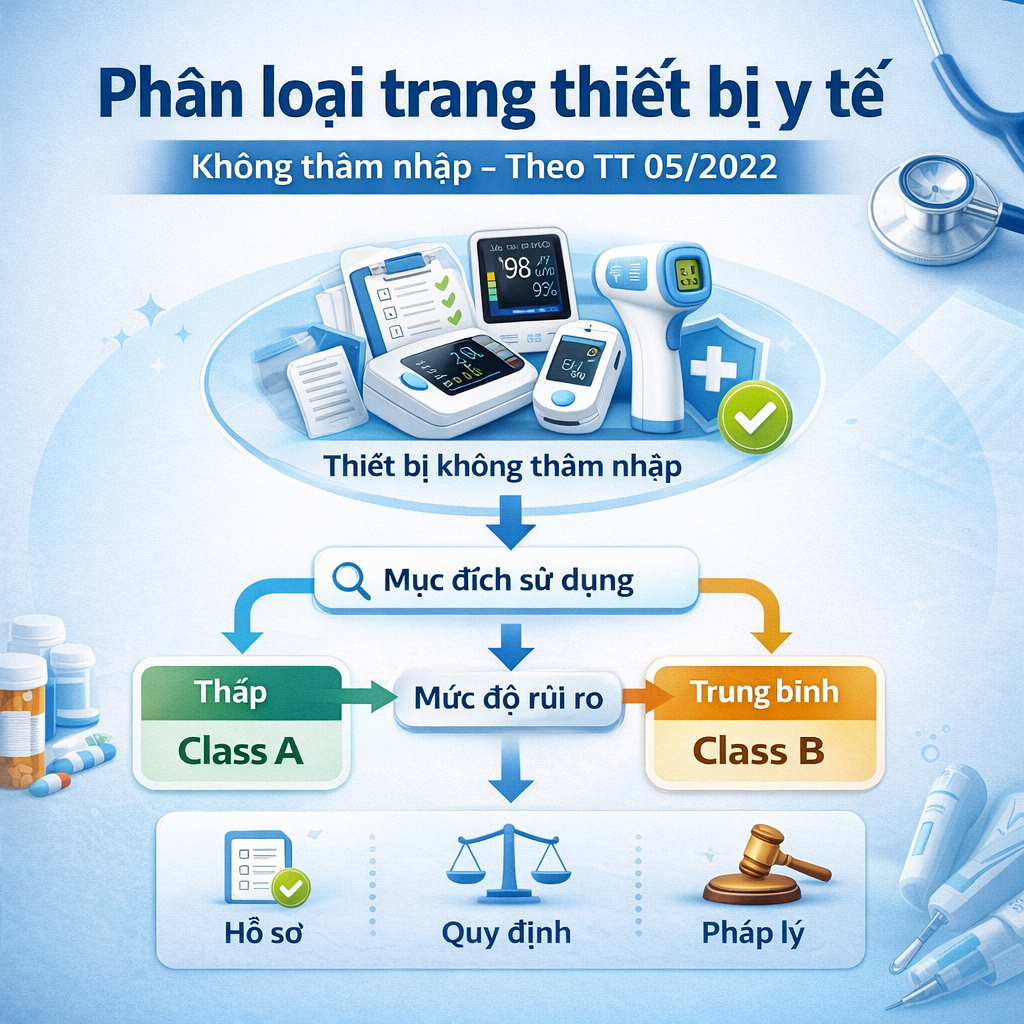
This article will help you clearly understand the Non-invasive Medical Device group.
1. Overview of Medical Device Classification
Under current Vietnamese regulations, medical devices are classified into four categories based on their potential risk arising from design, manufacturing, and intended use:
-
Class A: Low risk
-
Class B: Low–moderate risk
-
Class C: Moderate–high risk
-
Class D: High risk
The applicable classification rule depends on the intended purpose, duration of contact, and degree of invasiveness to the human body.
2. Definition: What Are Non-Invasive Medical Devices?
According to Circular No. 05/2022/TT-BYT, non-invasive medical devices are devices that:
-
Do not penetrate the body through natural body orifices;
-
Do not penetrate the body through the skin surface.
Note:
A non-invasive device may still be classified as a higher-risk device if it alters the biological composition of blood or comes into contact with serious open wounds.
3. Classification Rules for Non-Invasive Medical Devices
(Rules 1–4)
Based on Appendix I of Circular No. 05/2022/TT-BYT, non-invasive medical devices are classified under the following four rules:
Rule 1: Devices in Contact with Injured Skin
Applicable to devices such as wound dressings and wound cleansing products:
-
Class A:
Used for mechanical protection, compression, absorption of exudates, or as a simple mechanical barrier. -
Class B:
Used on superficial open wounds and intended to control the wound’s micro-environment. -
Class C:
Intended primarily for deep wounds (penetrating into the dermis or subcutaneous tissue), or specialized wounds that require healing by secondary intention (e.g. deep hemostatic dressings).
Rule 2: Devices for Channeling or Storing Blood, Tissues, or Body Fluids
Applicable to devices used to conduct, transfer, or store blood, body fluids, substances, or gases intended to be introduced into the body:
-
Class A:
Used for channeling or storing body fluids, tissues, substances, or gases for the final purpose of infusion, ingestion, or introduction into the body
(e.g. urine drainage tubing, specimen containers). -
Class B:
-
Connected directly to an active medical device classified as Class B or higher; or
-
Used to channel/store blood or other body fluids; or
-
Used for storing organs, tissues, or cells.
-
-
Class C:
Blood bags.
Rule 3: Devices with Biochemical Conversion Functions
Applicable to devices intended to modify the biological or chemical composition of blood, body fluids, or other liquids before being introduced into the body:
-
Class C:
All devices performing such functions
(e.g. dialysis membranes, blood centrifuges used to separate plasma for reinfusion). -
Exception – Class B:
If the modification only involves filtration, centrifugation, or simple exchange of gas or heat, depending on the specific case.
Rule 4: Other Non-Invasive Medical Devices
This is a general rule applicable to devices not covered under Rules 1–3:
-
Class A:
Devices that do not come into contact with the patient or only contact intact skin
(e.g. hospital beds, wheelchairs, mechanical blood pressure monitors, standard medical cotton and bandages). -
Class B:
-
Devices used to contain blood, gases, tissues, or body fluids (e.g. blood bags);
-
Devices used to clean, disinfect, or sterilize contact lenses or invasive medical devices.
-
4. Why Choose Songwin International Logistics Vietnam?
Incorrect classification (e.g. confusion between Class A and B, or B and C) can result in incorrect conformity declaration or registration procedures, causing cargo congestion at ports and unnecessary storage and demurrage costs.
At Songwin International Logistics Vietnam, we are not just a logistics provider — we are your strategic partner in solving medical device regulatory challenges.
Our Core Strengths:
-
Accurate classification consulting
Our experts have in-depth knowledge of Circular 05/2022/TT-BYT and related regulations (Decree 98/2021/ND-CP). -
End-to-End Logistics Services (Door-to-Door)
International transportation and professional customs clearance for medical devices, consumables, and diagnostic reagents. -
Comprehensive regulatory support
Import license application, circulation registration, and conformity declaration for Class A and B devices. -
Cost & time optimization
Minimizing compliance risks and ensuring on-time delivery to support your business operations.
Songwin International Logistics – Strong in Compliance, Fast in Transportation.
👉 Contact us today for a FREE medical device classification consultation!
SONGWIN INTERNATIONAL LOGISTICS VIETNAM CO., LTD.
📍 Address: 344 Nguyen Trong Tuyen Street, Tan Son Hoa Ward, Ho Chi Minh City, Vietnam
📞 24/7 Consulting Hotline: +84 83 681 3969 – +84 373 262 105
📧 Email for document submission: Sales2@songwinlog.com
Songwin Logistics – Trust Builds the Brand, Professionalism Creates Success.