The Declaration of Applied Standards is a mandatory legal procedure for medical devices (MDs) classified as Class A and Class B to be lawfully placed on the Vietnamese market.
However, preparing the dossier in accordance with Article 26 of Decree No. 98/2021/NĐ-CP often causes confusion for many enterprises due to the complexity of legal documentation.
This article provides a clear breakdown of the 09 required documents, guidance on how to prepare them, and critical “must-know” notes to help your dossier be approved quickly and smoothly.
Overview of the Dossier
Under current regulations, a complete dossier consists of 09 document categories. Below is a detailed explanation of each required document.
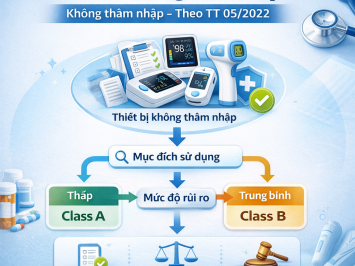
1. Declaration of Applied Standards
This is the official declaration document submitted by the enterprise to the Department of Health.
-
It includes information about the declaring entity and basic details of the medical device.
-
Must be prepared according to the latest template under Circular No. 44/2025/TT-BYT:
-
Form No. 02: for Class A medical devices
-
Form No. 03: for Class B medical devices
-
-
Prepared, signed, and sealed by the declaring entity (Vietnam-based enterprise).
Purpose:
To enable regulatory authorities to correctly classify and manage the device based on its risk level.
2. Quality Management System Certificate (ISO 13485)
This certificate proves that the manufacturing facility complies with the medical device–specific quality management system.
Important notes:
-
The ISO 13485 certificate must be valid at the time of submission.
-
For imported devices, a certified true copy is required.
Issued by:
The manufacturer / legal owner (certified by a conformity assessment body).
Purpose:
To ensure the product is manufactured under a stable and controlled quality system.
3. Letter of Authorization (LoA)
A legal document authorizing the Vietnamese enterprise to represent the product owner in registering and marketing the medical device.
Important notes:
-
For imported products:
-
Must be consular legalized in the country of origin.
-
Must be translated into Vietnamese and notarized.
-
-
Must be valid at the time of submission.
Issued by:
The medical device owner / manufacturer.
Purpose:
To confirm intellectual property rights and lawful distribution, preventing counterfeit or unauthorized products.
4. Warranty Eligibility Confirmation Letter
A written commitment from the product owner confirming that the Vietnamese entity has sufficient capability (personnel and technical resources) to provide warranty services.
Exception:
Not required for single-use medical devices (this must be clearly stated in the technical documentation).
Issued by:
The medical device owner (often issued together with the LoA).
Purpose:
To protect consumer rights after purchase.
5. Technical Summary Description
A detailed description of the device, including:
-
Structure
-
Operating principles
-
Functions
-
Technical specifications
Requirements:
-
Must be prepared in Vietnamese.
-
Content must be consistent with the original Technical File provided by the manufacturer.
Prepared by:
The declaring enterprise, based on documents issued by the device owner.
Applicable template:
According to Appendix I of Decree No. 98/2021/NĐ-CP.
6. Certificate of Conformity or Applied Standards Declaration
This document specifies which quality standards the device complies with (e.g. Enterprise Standards – TCCS, IEC, ASTM, ISO, etc.).
Preparation notes:
-
Usually includes:
-
Enterprise Standards (TCCS) issued by the owner, along with the decision to promulgate the standards.
-
-
If international standards (IEC, ISO, etc.) are applied:
-
A comparison or equivalence table is required.
-
Issued by:
The medical device owner.
7. Instructions for Use (IFU)
User manuals or instructions covering:
-
Proper usage
-
Storage
-
Safety warnings
Requirements:
-
A Vietnamese version is mandatory.
-
For imported products:
-
Must be accurately translated from the original foreign-language version.
-
Prepared by:
The declaring enterprise (translated or compiled from the manufacturer’s documents).
Purpose:
To ensure safe and effective use of the medical device.
8. Product Label Samples
Design of:
-
Supplementary labels (for imported products), or
-
Primary labels (for domestically manufactured products)
Requirements:
-
Must comply with Decree No. 111/2021/NĐ-CP on Goods Labeling and Decree No. 98/2021/NĐ-CP.
-
Mandatory contents include:
-
Product name
-
Origin
-
Product owner
-
Circulation number, etc.
-
Prepared by:
The declaring enterprise.
9. Certificate of Free Sale (CFS)
A certificate proving that the product is legally marketed in the country of manufacture or reference countries.
Important notes:
-
Must be valid at the time of submission.
-
Requires consular legalization.
Issued by:
A competent authority in the exporting country.
Summary Table – Document Sources
| Document Type | Main Provider | Actions Required by VN Declaring Entity |
|---|---|---|
| 1. Declaration Form | VN Enterprise | Prepare according to Circular 44/2025 |
| 2. ISO 13485 | Manufacturer | Check validity, notarized copy |
| 3. Letter of Authorization | Product Owner | Consular legalization, translation |
| 4. Warranty Letter | Product Owner | Review content, translate if needed |
| 5. Technical Description | Product Owner (original) | Prepare Vietnamese version |
| 6. Applied Standards | Product Owner | Translate and certify |
| 7. IFU | Product Owner (original) | Translate into Vietnamese |
| 8. Label Samples | VN Enterprise | Design draft labels |
| 9. CFS | Foreign Authority | Consular legalization, translation |
Conclusion
Preparing a medical device declaration dossier requires absolute accuracy and consistency across all documents.
Even a minor discrepancy in product name, model number, or expiry date may lead to dossier rejection or requests for amendment, causing delays and additional costs.
Comprehensive Medical Device Compliance Solutions by Songwin Log
Are you struggling with device classification?
Are you concerned about dossier rejection due to errors in consular legalization or translation?
At Songwin Log, we understand that mastering Decree 98/2021/NĐ-CP and new regulations such as Circular 44/2025/TT-BYT is a major challenge for trading enterprises. That’s why we provide end-to-end solutions to help you bring your products to market faster and more cost-effectively.
Why Choose Songwin Log?
Deep Expertise in Medical Devices
Our specialists have in-depth knowledge of the entire process—from risk classification (A, B, C, D) to declaration and circulation registration. We are committed to achieving first-time approval.
All-in-One Service (Legal + Logistics)
Beyond documentation, Songwin Log is a professional logistics provider, offering:
-
Licensing services (Declaration / Circulation Registration)
-
International transportation (Sea & Air)
-
Specialized customs clearance for medical devices
-
Domestic delivery to your warehouse
Handling Complex Cases
We specialize in incomplete dossiers, label corrections, urgent consular legalization, and other challenging scenarios.
Transparent & Cost-Effective
All-inclusive pricing with no hidden costs. We advise the most beneficial options for your business (import duties, VAT optimization, etc.).
Songwin Log Support Process
Step 1: Receive information & preliminary dossier assessment (Free of charge)
Step 2: Advise on classification and required documents
Step 3: Draft, translate, and complete the dossier on behalf of the client
Step 4: Submit the dossier, monitor progress, and deliver results
Step 5: Post-approval consultation (customs clearance when goods arrive)
📞 Contact us now for free 1:1 consultation
Don’t let administrative procedures slow down your business opportunities.
Let Songwin Log be your trusted legal and import–export partner.
Hotline/Zalo: +84 836 813 969 – +84 373 262 105
Email: sales2@songwinlog.com
Address: 344 Nguyen Trong Tuyen Street, Tan Son Hoa Ward, Ho Chi Minh City, Vietnam
Songwin Log – Strong Compliance, Fast Logistics