In the context of the rapidly growing healthcare market, placing medical devices on the Vietnamese market requires enterprises to strictly comply with legal regulations. One of the most important procedures for Class A and Class B medical devices is the Declaration of Applicable Standards.
This article provides a comprehensive overview of this procedure based on the most current legal frameworks, including Decree 98/2021/NĐ-CP, Circular 05/2022/TT-BYT, and Circular 44/2025/TT-BYT.
1. What is the Declaration of Applicable Standards and who does it apply to?
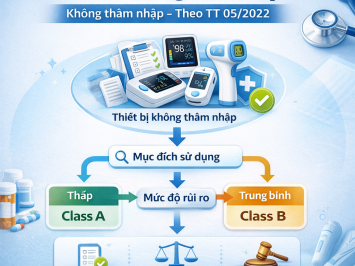
According to Decree 98/2021/NĐ-CP, medical devices in Vietnam are classified into four risk levels: A, B, C, and D.
-
Declaration of Applicable Standards: Mandatory for Class A and Class B medical devices.
-
Registration for Circulation: Applicable to Class C and Class D medical devices.
Therefore, if your company manufactures, trades, or imports Class A or B medical devices, you are required to complete the Declaration of Applicable Standards before placing the products on the Vietnamese market.
2. Conditions for Declaration
(In accordance with Decree 98/2021/NĐ-CP)
The declaring entity must meet the following conditions:
-
Have a legally established business presence in Vietnam.
-
Possess a valid Letter of Authorization from the medical device owner (except where the owner is based in Vietnam and declares directly).
-
Hold a valid ISO 13485 Quality Management System Certificate.
-
Ensure appropriate warranty facilities and qualified technical personnel.
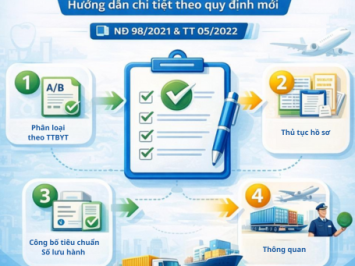
3. Dossier for Declaration of Applicable Standards
This is the most critical part of the procedure and also where errors most frequently occur. According to Article 26 of Decree 98/2021/NĐ-CP, the dossier includes:
-
Declaration Form for Applicable Standards (Class A or B):
Using Form No. 02 or 03 as stipulated in Circular 44/2025/TT-BYT. -
ISO 13485 Certificate (valid).
-
Letter of Authorization (LoA) from the medical device owner to the declaring entity
(Consular legalization required for imported devices). -
Warranty Eligibility Certificate, except for single-use medical devices.
-
Technical Summary Description:
In Vietnamese, accompanied by technical documents issued by the device owner, describing functions and specifications. -
Certificate of Conformity or the standards applied as declared by the device owner.
-
Instructions for Use (IFU) in Vietnamese.
-
Product Label Samples intended for circulation in Vietnam.
-
Valid Free Sale Certificate (CFS) at the time of submission.
-
Other supporting documents (if any).
4. Declaration Procedure
Currently, this procedure is conducted entirely online via the National Online Public Service System for Medical Device Management.
Step 1: Create a corporate account on the system.
Step 2: Upload the complete dossier.
Step 3: The Department of Health where the company is headquartered receives the dossier.
Step 4: Once the dossier is deemed valid, the Department of Health issues a Receipt Acknowledgement.
➡️ The Declaration Number is the number stated on this receipt.
Step 5: The information is publicly disclosed on the official portal.
Important Note:
The enterprise bears full legal responsibility for the accuracy of the dossier.
Incorrect classification (e.g., intentionally classifying a Class C device as Class A to expedite procedures) may result in severe penalties, including revocation of the circulation number.
A Comprehensive Solution for Your Business
Are you struggling with medical device classification under Circular 05?
Are you concerned about preparing technical documentation or consular legalization of foreign documents?
Let Songwin Logistics accompany you.
With extensive experience in handling thousands of dossiers under Decree 98, we provide end-to-end solutions that are Fast – Accurate – Fully Compliant.
Why Choose Songwin Logistics?
✔ Deep Expertise
Our specialists have in-depth knowledge of Decree 98/2021 and Circular 05/2022, ensuring 100% accurate classification, minimizing risks of post-inspection and revocation.
✔ Time Optimization
We streamline dossier preparation and standardization, and efficiently handle issues arising on the DMEC system.
✔ One-Stop Service Package
-
Medical device classification consultancy (A, B, C, D).
-
Drafting and reviewing declaration dossiers (ISO, CFS, Authorization, etc.).
-
Certified translation and consular legalization.
-
Representation in dossier submission and liaison with the Department of Health.
-
Post-approval consultancy: post-market inspection, advertising compliance, and price declaration regulations.
✔ Transparent Pricing
One-time quotation with a commitment to no hidden costs.
Our Working Process
-
Information Intake: Preliminary legal assessment of the product.
-
Contract Signing: Clear commitments on timelines and outcomes.
-
Dossier Completion: Preparation of all required documents for client review and signature.
-
Result Delivery: Issuance of the Receipt for Declaration of Applicable Standards.
Don’t let administrative barriers delay your business plan.
CONTACT US NOW FOR A FREE CONSULTATION:
📞 Hotline/Zalo: 083.681.3969 – 0373.262.105
📧 Email: sales2@songwinlog.com
🏢 Address: 344 Nguyen Trong Tuyen Street, Tan Son Hoa Ward, Ho Chi Minh City, Vietnam