Importing medical devices is one of the most strictly regulated sectors in Vietnam. A thorough understanding of the regulations stipulated in Decree No. 98/2021/NĐ-CP and Circular No. 05/2022/TT-BYT is a prerequisite for businesses to ensure smooth customs clearance, avoid storage costs, and prevent administrative penalties.
This article provides a comprehensive overview of the current procedures for importing medical devices into Vietnam.
1. Legal Framework Governing Medical Device Imports
When importing medical devices, enterprises must comply with the following key legal documents:
-
Decree No. 98/2021/NĐ-CP: Regulates the management of medical devices, including classification, manufacturing, circulation, import, and export.
-
Circular No. 05/2022/TT-BYT: Provides detailed guidance for implementing certain provisions of Decree 98, particularly the list of medical devices subject to import licensing requirements.
2. Medical Device Classification (The Most Critical Step)
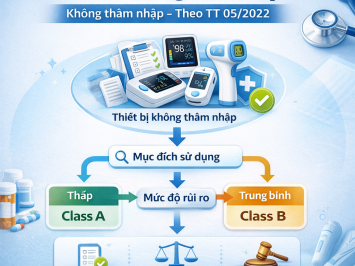
Under Decree 98/2021/NĐ-CP, the import procedure for medical devices depends on their risk classification. Medical devices are categorized into four classes:
-
Class A (Low risk)
Examples: Cotton and bandages, conventional medical gloves, basic hospital beds. -
Class B (Low–medium risk)
Examples: Catheters, syringes, needles. -
Class C (Medium–high risk)
Examples: X-ray machines, diagnostic imaging equipment. -
Class D (High risk)
Examples: Implantable medical devices, pacemakers.
Note: Classification must be conducted by an organization that is legally qualified and certified by the Ministry of Health to perform medical device classification.
3. Import Conditions and Procedures
Depending on the classification result (A, B, C, or D), enterprises must follow different regulatory procedures:
Case 1: Class A and Class B Medical Devices
Enterprises are required to conduct a Declaration of Applied Standards.
-
Result: Acknowledgement Receipt of the Declaration of Applied Standards issued by the Department of Health.
-
Customs clearance: When importing, enterprises only need to present:
-
The acknowledgement receipt, and
-
The medical device classification result.
-
Case 2: Class C and Class D Medical Devices
Enterprises must apply for Marketing Authorization (Circulation Registration).
-
In cases where the device does not yet have a marketing authorization number but falls under the categories specified in Circular 05/2022/TT-BYT, an Import License is required.
-
Typical cases requiring an import license include:
-
Import for research or testing purposes
-
Humanitarian aid or donations
-
Special-purpose use without a circulation number
-
Summary of Required Legal Documents Before Shipment Arrival
Medical device classification result
Marketing authorization number (for Class C, D) or acknowledgement receipt of applied standards declaration (for Class A, B)
Import license (if applicable)
4. Customs Clearance Dossier for Medical Device Imports
In addition to standard commercial documents, enterprises must prepare the following specialized regulatory documents for customs clearance:
-
Customs declaration (E-Customs system)
-
Commercial invoice
-
Packing list
-
Bill of Lading / Airway Bill
-
Legal documents:
-
Medical device classification result
-
Marketing authorization certificate or acknowledgement receipt of applied standards declaration
-
Import license (if applicable)
-
-
Technical documents: Catalogue, Instructions for Use (may be required by customs for HS code verification)
-
Certificate of Origin (C/O), if tariff preferences are claimed
5. Taxation and Labeling Considerations
-
Value Added Tax (VAT):
Under current regulations, many specialized medical devices are eligible for preferential VAT rates (commonly 5%) or may be VAT-exempt, depending on the applicable period and HS code. Enterprises should carefully review the applicable tax policies to optimize costs. -
Labeling requirements:
Imported goods must have original labels and Vietnamese supplementary labels, clearly indicating:-
Product name
-
Country of origin
-
Manufacturer information
-
Importer information
-
Marketing authorization number or import license number
-
COMPREHENSIVE LOGISTICS SOLUTIONS FOR MEDICAL DEVICES BY SONGWIN INTERNATIONAL LOGISTICS
Importing medical devices requires absolute accuracy in documentation and in-depth knowledge of pharmaceutical and medical device regulations. Even a minor error in classification or HS code determination can result in shipment delays, high storage costs, and disruptions to hospital or clinic projects.
Songwin International Logistics Vietnam is proud to be a leading provider of specialized logistics solutions for the healthcare sector.
Why Choose Songwin?
-
In-depth Regulatory Expertise
Our expert team has a thorough understanding of Decree 98/2021/NĐ-CP and Circular 05/2022/TT-BYT, providing accurate consultancy on import licensing, Class A/B declarations, and Class C/D circulation registration. -
Accurate Classification & HS Code Consultancy
We assist clients in reviewing technical documentation and selecting the correct HS code to optimize import duties and VAT in full compliance with regulations. -
Multimodal Transportation Solutions
With a global agent network, Songwin safely transports high-value medical devices, oversized machinery (MRI, CT scanners), and temperature-controlled goods (reagents) from factory warehouses (EXW) to customers’ facilities in Vietnam (DDP). -
Flexible Issue Resolution
We provide fast and effective support to resolve issues arising at ports and airports.
Core Services
-
End-to-end customs clearance services for medical devices
-
International sea freight and air freight
-
Consultancy for import licensing and medical device declaration/registration
Don’t let complex customs procedures delay your business opportunities.
Let Songwin International Logistics be your trusted partner in bringing medical devices into Vietnam — Fast, Safe, and Fully Compliant.
CONTACT US FOR A FREE CONSULTATION:
-
SONGWIN INTERNATIONAL LOGISTICS VIETNAM CO., LTD.
-
Hotline: +84 83 681 3969 – +84 373 262 105
-
Email: sales2@songwinlog.com
-
Address: 344 Nguyen Trong Tuyen Street, Tan Son Hoa Ward, Ho Chi Minh City, Vietnam