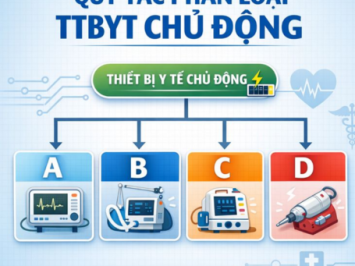
DETAILED GUIDANCE: RULES FOR CLASSIFICATION OF ACTIVE MEDICAL DEVICES UNDER CIRCULAR 05/2022/TT-BYT
Date Submitted: 26/01/2026 01:57 PM
Xem chi tiết

GUIDELINES FOR CLASSIFICATION OF INVASIVE MEDICAL DEVICES (According to Circular No. 05/2022/TT-BYT)
Date Submitted: 23/01/2026 02:49 PM
Xem chi tiết
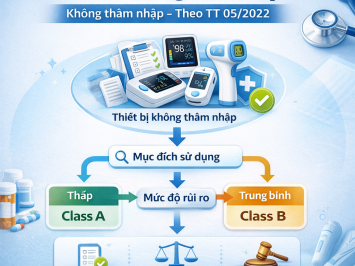
DETAILED GUIDELINES: CLASSIFICATION RULES FOR NON-INVASIVE MEDICAL DEVICES (In accordance with Circular No. 05/2022/TT-BYT)
Date Submitted: 21/01/2026 02:43 PM
Xem chi tiết

CLASSIFICATION OF MEDICAL DEVICES According to Decree No. 98/2021/ND-CP & Circular No. 05/2022/TT-BYT
Date Submitted: 19/01/2026 11:48 AM
Xem chi tiết

Declaration of Applied Standards – Mandatory Procedure for Class A & B Medical Devices
Date Submitted: 16/01/2026 02:02 PM
Xem chi tiết
GUIDELINES ON DECLARATION OF APPLICABLE STANDARDS FOR MEDICAL DEVICES
Date Submitted: 14/01/2026 10:53 AM
Xem chi tiết
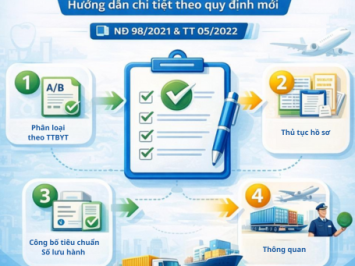
DETAILED GUIDELINES ON IMPORT PROCEDURES FOR MEDICAL DEVICES
Date Submitted: 12/01/2026 04:19 PM
Xem chi tiết

LATEST IMPORT PROCEDURES FOR DIETARY SUPPLEMENTS & FULL-SERVICE SOLUTIONS FROM SONGWIN LOGISTICS
Date Submitted: 09/01/2026 11:58 AM
Xem chi tiết

DETAILED EXPORT PROCEDURE & SOLUTIONS FROM A 10-YEAR INDUSTRY EXPERT
Date Submitted: 07/01/2026 03:23 PM
Xem chi tiết
What is a Bill of Lading (B/L)?
Date Submitted: 05/01/2026 11:44 AM
Xem chi tiết






















